Hastelloy G-30 alloy (UNS N06030) is a nickel-chromium-iron material with high resistance to wet phosphoric acid (P2O5). It was invented in the 1970s and 1980s and has been in use for more than 30 years.
Chemical Composition Of G-30 Alloy
Due to its high chromium content, G-30 alloy is very resistant to other oxidising acids. Due to its significant molybdenum and copper content, it is very resistant to hydrochloric and sulphuric acids. G-30 alloy is also resistant to fairly moderate chloride-induced local attack. This may account for the deposits below in evaporators used to concentrate P2O5.
G-30 alloy is specifically designed to resist commercial phosphoric acid attack. The alloy is particularly successful in heat exchange tubes and other components in the fertiliser industry. Its high cobalt content also makes it the alloy of choice for pickling of strongly oxidising solutions.
G-30 plate conforms to ASME SB 582/ASTM B 582. Bar stock is available in ASME SB 581/ASTM B 581.
| Peso % | |
| Níquel | 43 Balance |
| Crómio | 30 |
| Iron | 15 |
| Molybdenum | 5.5 |
| Tungsten | 2.5 |
| Copper | 2 |
| Niobium | 0.8 |
| Cobalt | 5 max. |
| Manganês | 1.5 max. |
| Silício | 0.8 max. |
| Carbono | 0.03 max. |
Wet Process Phosphoric Acid
Wet phosphoric acid (P2O5) comes from the reaction of phosphate ore with sulphuric acid.
It contains many impurities and has a P2O5 concentration of only about 30 percent. This is because a large amount of rinse water is required to separate it from the other main reaction product, calcium sulphate. Typical impurities include unreacted sulphuric acid, various metal ions, fluoride ions and chloride ions. Fluoride ions tend to form complexes with metal ions and are therefore less abundant than chloride ions. This strongly influences the electrochemical reaction between the “Wet Process” phosphoric acid and the metal material. Particulate matter can also be present in the “Wet Process” acid.
Typically, the P2O5 concentration is increased to 54 percent in this process. The concentration of impurities decreases with increasing phosphoric acid concentration. This counteracts the concentration effect of acid corrosivity.
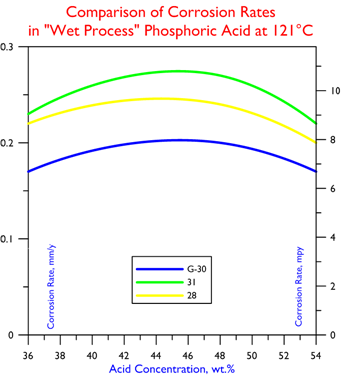
The chart below, comparing G-30 alloy to stainless steel. Corrosion data based on testing of three different concentrations (36, 48, and 54%) of “Wet Process” phosphoric acid at 121°C (250°F).
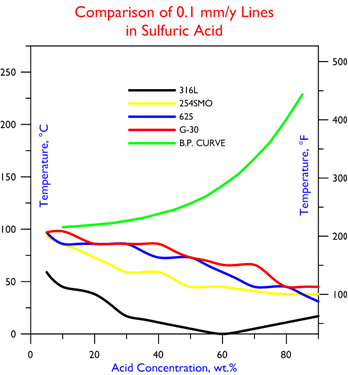
Resistant to hydrochloric acid and sulfuric acid, the corrosion resistance data of G-30 is as follows.
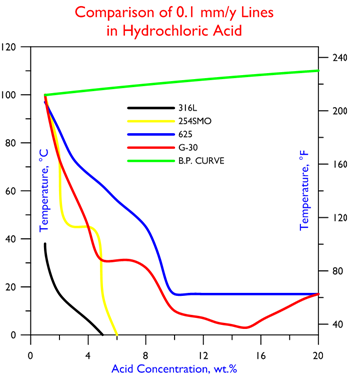
Comparative performance data for hydrochloric acid corrosion resistance are as follows.